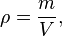The mass density or density of a material is its mass per unit volume. The symbol most often used for density is ρ (the lower case Greek letter rho). Mathematically, density is defined as mass divided by volume:[1]
Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure but certain chemical compounds may be denser.
Less dense fluids float on more dense fluids if they do not mix. This concept can be extended, with some care, to less dense solids floating on more dense fluids. If the average density (including any air below the waterline) of an object is less than water it will float in water and if it is more than water it will sink in water.
http://xenontics.blogspot.com/